General Overview of Research lines
Thermophysical properties of Nanofluids
This research is basically focussed on the characterization and development of novel materials which increase the heat transfer in conventional installations of heating and cooling, looking forward more efficient energy transference and the reduction of energy losses by friction or wear.
To accomplish this , the thermal conductivity is a property that plays a key role as the main factor influencing the process of heat transfer by convection in fluids. The heat transfer fluids used at present (water , ethylene glycol ...) exhibit extremely poor thermal conductivity and heat storage capacity.
It was been recently observed that an effective way to improve thermal conductivity is the suspension of small solid particles in a conventional base fluid , in order to improve its transport properties, flow characteristics and thermal stability .
The solid particles selected in this case are the so-called nanoparticles, i.e. particles of average size smaller than 100 nm , which minimize problems as sedimentation and the tendency to occlusion observed for microscopically scale sized particles.
We selected as base fluids water and ethylene glycol , since these fluids are the most widely used as heat transfer fluids in industry, and the metal oxide nanoparticles employed were the following : Al 2 O 3 , CuO, Fe x O y and ZnO.
These oxides have been either purchased commercially or synthesized to control their size and shape. The synthetic route used has always been the co - precipitation.
This work aimed to make a contribution to the establishment of methods of nanofluid sample characterization, and further precise measurement of density , viscosity, thermal conductivity and rheological profile of various samples of nanofluids , in the broader ranges of temperature and pressure accessible.
Antioxidant distribution in model food emulsions
Emulsions, or macroemulsions, represent a major group of colloidal systems which are present either as end product or during the processing of products in a huge range of technological areas including alimentary, agrochemical, pharmaceutical, cosmetic, paint and oil industries, etc
What makes emulsions so attractive? The main reason is because they are suitable solvents not only for water-soluble but also for hydrophobic substances. Many foods present emulsified form and contain lipids, which can degrade due to the presence of oxygen ( these reactions are called auto-oxidation reactions ), producing undesired odour and flavour in food , and diminishing its nutritional value.
With the object to minimize lipid degradation antioxidants are readily added to food. In general an antioxidant reacts with the free radical , yielding a hydrogen and regenerating the initial lipid .
What factors affect this activity?
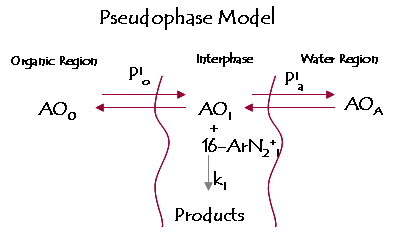
The antioxidant distribution between two immiscible liquids is represented by its partition constant P A O , depending on the concentration of the molecule in each phase , which is easy to determine using a variety of techniques after extracting an aliquot of each phase. But the distribution of molecules in emulsified systems is more complex due to the existence of multiple possibilities of balance .
In an emulsion there are 3 regions, namely organic , interfacial and aqueous , and the antioxidant is distributed in the three phases, depending on the partition constant of the interfacial, aqueous, and organic interphase . Existing distribution in emulsions studied to date are based upon rupture, determining the antioxidant in organic and aqueous phase , - an P A O value- , and since it is physically impossible to isolate the interface, P A O value provides no topographical information about the location of the antioxidant in the system nor give any information on the fraction of molecules found in each of the three regions.
This research was aimed to determine the partition constants of a variety of antioxidants between the different regions of model emulsified systems, intended to estimate their distribution and to analyse the effects of relevant experimental parameters (temperature, acidity, hydrophobicity of the antioxidant, nature of the oil, etc.) on their distributions. In addition, establishing a solid experimental and methodological background to determine antioxidant distributions in more complex and/or food-like emulsions was also a crucial objective.
For references, see the Publications page.