2017 2016 2015 2014 2013 2012 2011 2010 2009 2008 2006 2005 2004 2017
35. Carolina Hermida Merino; Martin Perez Rodriguez; Manuel Martinez Piñeiro; Maria Jose Pastoriza Gallego* . “Tuning the electrical conductivity of exfoliated graphite nanosheets nanofluids by surface functionalization”. Soft Matter , 2017, 13 , 3395-3403
34. Margarida L. Ferreira María J. Pastoriza-Gallego João M. M. Araújo José N. Canongia Lopes Luís Paulo N. Rebelo Manuel M. Piñeiro Karina Shimizu Ana B. Pereiro . “ Influence of Nanosegregation on the Phase Behavior of Fluorinated Ionic Liquids ”. J. Phys. Chem. C , 2017 , 121 (9), pp 5415–5427
2016
33. Carolina Hermida Merino; Martin Perez Rodriguez; Manuel Martinez Piñeiro; Maria Jose Pastoriza Gallego*. “Evidence of viscoplastic behaviour of exfoliated graphite nanofluids”. Soft Matter . 2016 . DOI: 10.1039/C5SM02932E.
2015
32. Carlos Bravo-Díaz, Laurence S. Romsted, Changyao Liu, Sonia Losada-Barreiro, Maria José Pastoriza-Gallego, Xiang Gao, Qing Gu, Krishnan Gunaseelan, Verónica Sánchez-Paz, Yongliang Zhang and Aijaz Dar.To Model Chemical Reactivity in Heterogeneous Emulsions:Think Homogeneous Microemulsions. Langmuir 2015 , 31 (33 ), 8961-8979
31. María José Pastoriza-Gallego, Sonia Losada-Barreiro, Carlos Bravo-Díaz. Interfacial kinetics in octane based emulsions. Effects of surfactant concentration on the reaction between 16-ArN 2 + and octyl and lauryl gallates. Colloids and Surfaces A: Physicochemical and Engineering Aspects . 2015, doi:10.1016/j.colsurfa.2014.10.019 .
30. D. Cabaleiro ,J. Nimo, M.J. Pastoriza-Gallego , M.M. Piñeiro , J.L. Legido , L. Lugo. Thermal conductivity of dry anatase and rutile nano-powders and ethylene and propylene glycol-based TiO 2 nanofluids. J. Chem. Thermodynamics . 2015, 83, 67–76.
29. Alejandra Mariano, María José Pastoriza-Gallego, Luis Lugo, Lelia Mussari , Manuel M. Piñeiro. Co3O4 ethylene glycol-based nanofluids: Thermal conductivity, viscosity and high pressure density. International Journal of Heat and Mass Transfer. 2015, 85, 54–60.
2014___ ____________ _ ___________________________________
________
alpha-Fe2O3________________________Fe3O4 _ __
Homogeneous and stable magnetic nanofluids containing iron oxide nanoparticles, a-Fe2O3 (hematite) and Fe3O4 (magnetite) in ethylene glycol, were prepared at concentrations up to 25% in mass fraction.Commercial Hexagonal Scalenohedral-shaped a-Fe2O3 nanoparticles were selected while Fe3O4 nanoparticles were synthesized using a coprecipitation method.The products were characterized by transmission and scanning electron microscopy and x-ray diffraction. The thermal conductivity of both nanofluids was measured as a function of volume fraction and temperature. The results illustrate that the enhanced thermal conductivity of the nanofluids increases with volume fraction but is temperature independent. The experimental results show that both types of nanoparticles in this base fluid present no significant aggregation. These experimental values were also compared with theoretical models. Moreover, the density of these nanofluids was measured as a function of volume fraction, temperature, and pressure. The volumetric behavior of nanofluids containing hematite is closer to the ideal behavior than those using magnetite.
28. María José Pastoriza-Gallego, Luis Lugo, David Cabaleiro, José Luis Legido, Manuel M Pineiro. Thermophysical profile of ethylene glycol-based ZnO nanofluids. J. Chem. Thermodynamics . 2014, 73, 23-30.
201327. Freitas, S.V.D. , E Silva, F.A. , Pastoriza-Gallego, M.J. , Piñeiro, M.M., Lima, A.S. , Coutinho, J.A.P. Measurement and prediction of densities of vegetable oils at pressures up to 45 MPa . J. Chem. Eng. Data. 2013, 58 (11), 3046-3053.
26. Pereiro, Ana; Pastoriza-Gallego, María José; Shimizu, Karina; Marrucho, Isabel; Canongia Lopes, Jose Nuno; Piñeiro, Manuel; Rebelo, Luis Paulo. On the Formation of a New, Third, Nanostructured Domain in Ionic Liquids. Journal of Physical Chemistry B. 2013 , 117 (37), 10826–10833. DOI: 10.1021/jp402300c
25.Pastoriza-Gallego, M.J. , Pérez-Rodríguez, M. , Gracia-Fernández, C. , Piñeiro, M.M. . Study of viscoelastic properties of magnetic nanofluids: An insight into their internal structure . Soft Matter . 2013, 9: 48, 11690-11698.
24. David Cabaleiro, Maria J. Pastoriza-Gallego, Carlos Gracia-Fernández, Manuel M. Piñeiro and Luis Lugo. Rheological and volumetric properties of TiO2-ethylene glycol nanofluids. Nanoscale Research Letters. 2013, 8 :286 doi:10.1186/1556-276X-8-286
23. D. Cabaleiro, M.J. Pastoriza-Gallego, M.M. Piñeiro, L. Lugo. Characterization and measurements of thermal conductivity, density and rheological properties of zinc oxide nanoparticles dispersed in water + ethylene glycol mixture . J. Chem. Thermodynamics. 2013, 58, 405–415
22. Alejandra Mariano, María José Pastoriza-Gallego, Luis Lugo, Alberto Camacho, Salvador Canzonieri, Manuel M. Piñeiro. Thermal conductivity, rheological behaviour and density of non-Newtonian ethylene glycol-based SnO2 nanofluids. Fluid Phase Equilibria. 2013, 337, 119-124
2012
21. Benigno Barbés, Ricardo Páramo, Eduardo Blanco, María José Pastoriza- Gallego, Manuel M. Piñeiro, José Luis Legido & Carlos Casanova. Thermal conductivity and specific heat capacity measurements of Al2O3 nanofluids. J Therm Anal Calorim. 2012, DOI 10.1007/s10973-012-2534-9
20. Pastoriza-Gallego, María José; Losada-Barreiro, Sonia; Bravo-Díaz, Carlos. Effects of acidity and emulsifier concentration on the distribution of Vitamin C in a model food emulsion. Journal of Physical Organic Chemistry . 2012, DOI: 10.1002/poc.2949
19. D. Cabaleiro, M.J. Pastoriza-Gallego, M.M. Piñeiro, J.L. Legido, L. Lugo. Thermophysical properties of (diphenyl ether + biphenyl) mixtures for their use as heat transfer fluids. J. Chem. Thermodynamics. 2012, 50, 80-88
_____________________________________________________
Experimental measurements of density, viscosity and thermal conductivity are reported for pure diphenyl ether and three different binary mixtures of diphenyl ether and biphenyl including the eutectic point. Density has been measured for the liquid phase at temperatures ranging from (298.15 to 363.15) K and for pressures up to 45 MPa using a high-pressure vibrating tube densimeter. A Tammann–Tait correlation of the experimental densities has been proposed for each composition. From these correlations, isothermal compressibility, isobaric thermal expansivity and internal pressure have been determined. Moreover, viscosity and thermal conductivity were experimentally determined at atmospheric pressure for several temperatures by using a rolling ball viscometer and a device based in the hot-wire technique, respectively. All the experimental devices used to determine the thermophysical properties were checked finding good agreements with previous literature data. The experimental viscosity values were correlated using the Vogel–Fulcher–Tammann, Avramov–Milchev and MYEGA equation.
2011
18. M. J. Pastoriza-Gallego, L. Lugo, J. L. Legido, M. M. Piñeiro. Rheological non-Newtonian behaviour of ethylene glycol-based Fe2O3 nanofluids.Nanoscale Res. Lett , 2011, 6:560, 1-7.17. M. J. Pastoriza-Gallego, L. Lugo, J. L. Legido, M. M. Piñeiro.Enhancement of thermal conductivity and volumetric behaviour of FexOy nanofluids. Journal of Applied Physics 2011, 110, 014309 1-9.
16. M. J. Pastoriza-Gallego, L. Lugo, J. L. Legido, M. M. Piñeiro.Thermal Conductivity and Viscosity Measurements of Ethylene Glycol-Based Al2O3 Nanofluids. Nanoscale Res. Lett, 2011, 6(1), 221 1-11.
15. Luciana I. N. Tome,Ramesh L. Gardas, Pedro J. Carvalho,Maria Jose Pastoriza-Gallego, Manuel M. Piñeiro,§ and Joao A. P. Coutinho. Measurements and Correlation of High-Pressure Densities of Phosphonium Based Ionic Liquids. J. Chem. Eng. Data, 2011, 56, 2205-2217.
___________________ __________________________________
The dispersion and stability of nanofluids obtained by dispersing Al2O3 nanoparticles in ethylene glycol have been analyzed at several concentrations up to 25% in mass fraction. The thermal conductivity and viscosity were experimentally determined at temperatures ranging from 283.15 K to 323.15 K using an apparatus based on the hot-wire method and a rotational viscometer, respectively. It has been found that both thermal conductivity and viscosity increase with the concentration of nanoparticles, whereas when the temperature increases the viscosity diminishes and the thermal conductivity rises. Measured enhancements on thermal conductivity (up to 19%) compare well with literature values when available. New viscosity experimental data yield values more than twice larger than the base fluid. The influence of particle size on viscosity has been also studied, finding large differences that must be taken into account for any practical application. These experimental results were compared with some theoretical models, as those of Maxwell-Hamilton and Crosser for thermal conductivity and Krieger and Dougherty for viscosity.
________________ _ ___________________________________
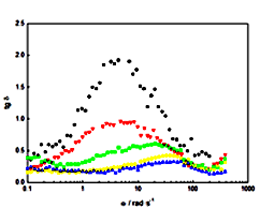
The rheological behaviour of ethylene glycol-based nanofluids containing hexagonal scalenohedral-shaped a-Fe2O3 (hematite) nanoparticles at 303.15 K and particle weight concentrations up to 25% has been carried out using a cone-plate Physica MCR rheometer. The tests performed show that the studied nanofluids present non-Newtonian shear-thinning behaviour. In addition, the viscosity at a given shear rate is time dependent, i.e. the fluid is thixotropic. Finally, using strain sweep and frequency sweep tests, the storage modulus G’, loss modulus G″ and damping factor were determined as a function of the frequency showing viscoelastic behaviour for all samples.
14. Maria Jorge Pratas, Mariana B. Oliveira, Maria Jose Pastoriza-Gallego,Antonio J. Queimada, Manuel M. Piñeiro, and Joao A. P. Coutinho. High-Pressure Biodiesel Density: Experimental Measurements, Correlation, and Cubic-Plus-Association Equation of State (CPA EoS) Modeling. Energy and Fuels 2011, 25 (8),:3806-3814 .
_____________________________________________________
Density is one of the most important biodiesel properties, because engine injection systems (pumps and injectors) must deliver an amount of fuel precisely adjusted to provide a proper combustion while minimizing greenhouse gas emissions. The pressure influence in fuel density has become particularly important with the increased use of modern common rail systems, where pressures can reach 250 MPa. Nevertheless, besides its importance, little attention has been given to high-pressure biodiesel densities. In fact, there are almost no reports in the literature about experimental high-pressure biodiesel density data. To overcome this lack of information, in this work, new experimental measurements, from 283 to 333 K and from atmospheric pressure to 45 MPa, were performed for methyl laurate, methyl myristate, and methyl oleate, for methyl biodiesels from palm, soybean, and rapeseed oils, and for three binary and one ternary mixture of these oils. Following previous works, where the cubic-plus-association equation of state (CPA EoS) was shown to be the most appropriate model to be applied to biodiesel production and purification processes, the new high-pressure experimental data reported here were also successfully predicted with the CPA EoS, with a maximum deviation of 2.5%. A discussion about the most appropriate CPA pure compound parameters for fatty acid methyl esters is also presented.
13. M. J. Pastoriza-Gallego, Verónica Sánchez-Paz, Laurence S. Romsted, Carlos Bravo-Díaz. Distribution of tert-butylhydroquinone in a corn oil/C12E6 / water based emulsion. Appication of the pseudophase kinetic model. Progress in Colloid and Polymer Science, 2011, 138, 33-38. ISBN 978-3-642-19037-7. Springer Heidelberg Dordrecht London New York.
12. M. J. Pastoriza-Gallego, C. Casanova, J. L. Legido, M. M. Piñeiro. CuO in water nanofluid: influence of particle size and polydispersity on volumetric behaviour and viscosity. Fluid Phase Equilibria 2011, 300, 188-196.
Top
2010
11. Alejandra Fernández-Alonso, M. J. Pastoriza-Gallego; Carlos Bravo-Diaz. Butanolysis of 4-methylbenzenediazonium ions in binary n-BuOH/H2O mixtures and in n-BuOH/SDS/H2O reverse micelles. Effects of solvent composition, acidity and temperature on the switch between heterolytic and homolytic dediazoniation mechanisms. Organic and Biomolecular Chemistry 2010, 8, 5304-5312.
Top
Abstract______________________________________________
The dispersion and stability of nanofluids obtained by dispersing CuO nanoparticles (obtained from different sources) in water have been analyzed. The volumetric behaviour up to high pressures (45 MPa), and atmospheric pressure viscosity were experimentally determined. It has been found that the influence of particle size in density is subtle but not negligible, but the differences in viscosity are very large and must be taken into account for any practical application. These viscosity differences can be described qualitatively by considering a theory describing either the aggregation state or the particle size distribution of the nanofluid
.______
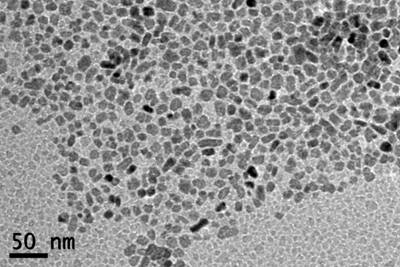
_________________a)_______________________________________ b)
Figure 5: TEM images of CuO nanoparticles in water (0.005% v/v) for a) S1 (CM20) and b) S2 (JEOL JEM-1010).
2009
10. M. J. Pastoriza-Gallego, C. Casanova, R. Páramo, B. Barbés, J. L. Legido, M. M. Piñeiro. A study on stability and thermophysical properties (density and viscosity) of Al2O3 in water nanofluid. Journal of Applied Physics 2009, 106(6), 064301 1-8.
9. Pastoriza-Gallego, Maria Jose; Sanchez-Paz, Veronica; Losada-Barreiro, Sonia; Bravo-Diaz, Carlos; Gunaseelan, K.; Romsted, Laurence S. Effects of Temperature and Emulsifier Concentration on a-Tocopherol Distribution in a Stirred, Fluid, Emulsion. Thermodynamics of a-Tocopherol Transfer between the Oil and Interfacial Regions. Langmuir 2009, 25(5), 2646-2653.
8. Pastoriza Gallego, M. Jose; Bravo-Diaz, Carlos. Butanolysis of 2-methylbenzenediazonium ions: product distribution, rate constants of product formation, and activation parameters. Journal of Physical Organic Chemistry 2009, 22(5), 390-396.
Top
2008
7. Sanchez-Paz, Veronica; Pastoriza-Gallego, Maria Jose; Losada-Barreiro, Sonia; Bravo-Diaz, Carlos; Gunaseelan, K.; Romsted, Laurence S. Quantitative determination of a-tocopherol distribution in a tributyrin/Brij 30/water model food emulsion. Journal of Colloid and Interface Science 2008, 320(1), 1-8.Abstract______________________________________________
The dispersion and stability of nanofluids obtained by dispersing Al2O3 nanoparticles obtained from different sources in water have been analyzed. The differences arising from different dispersion techniques, the resulting particle size distribution, and time stability among the different samples are evaluated. Then the volumetric behavior up to high pressures 25 MPa and atmospheric pressure viscosity were experimentally determined. It has been found that the influence of particle size in density is subtle but not negligible, but the differences in viscosity are very large and must be taken into account for any practical application. These viscosity differences can be rationalized by considering a theory describing the aggregation state of the nanofluid.
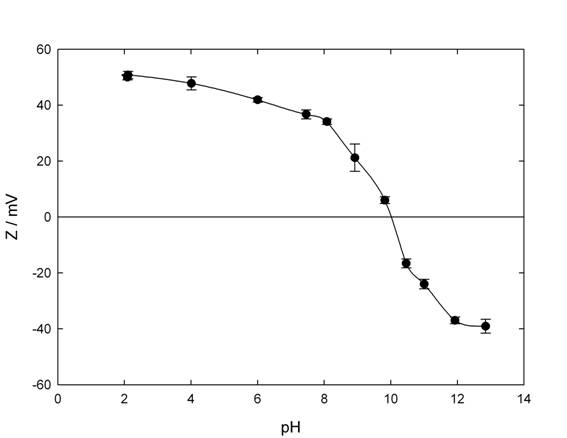
Figure 7: Influence of pH in Z potential for Al 2 O 3 / water nanofluids, (0.005%v/v), in 0.01M KCl solution.
6. Bravo-Diaz, Carlos; Pastoriza-Gallego, Maria Jose; Losada-Barreiro, Sonia; Sanchez-Paz, Veronica; Fernandez-Alonso, Alejandra. Dediazoniation of 1-naphthalenediazonium tetrafluoroborate in aqueous acid and in micellar solutions. International Journal of Chemical Kinetics 2008, 40(6), 301-309.
5. Sonia Losada-Barreiro, Verónica Sánchez-Paz, María José Pastoriza-Gallego, and Carlos Bravo-Díaz. Micellar Effects on the Reaction between an Arenediazonium Ion and the Antioxidants Gallic Acid and Octyl Gallate. Helvetica Chimica Acta 2008, 91, 21-34.
4. María José Pastoriza-Gallego, Alejandra Fernández-Alonso, Sonia Losada-Barreiro, Verónica Sánchez-Paz and Carlos Bravo-Díaz. Kinetics and mechanism of the reaction between 4-hexadecylbenzenediazonium ions and vitamin C in emulsions:further evidence of the formation of diazo ether intermediates in the course of the reaction. Advances Journal of Physical Organic Chemistry 2008, 21, 524-530.
Top
2006
3. K. Gunaseelan, Laurence S. Romsted, María José Pastoriza Gallego, Carlos Bravo-Díaz, Elisa González-Romero. Determining tocopherol distributions between the oil, water, and interfacial regions of macroemulsions: Novel applications of electroanalytical chemistry and the pseudophase kinetic model. Advances in Colloid and interface Science 2006, 123-126, 303-311.
Top
2005
2. María José Pastoriza Gallego, Carlos Bravo-Díaz, Elisa González-Romero. Dediazoniation in SDS/BuOH/H2O reverse micelles: structural parameters, kinetics, and mechanism of the reaction. Langmuir 2005, 21(7), 2675-2681.
Top
2004
1. María José Pastoriza Gallego, Carlos Bravo-Díaz, Elisa González-Romero. Fluorimetric determination of structural parameters of BuOH/SDS/H2O reverse micelles. Colloid and Surface A 2004, 249 (1-3), 25-28.
Top